Intramolecular C–H⋯F hydrogen bonding-induced 1,2,3-triazole-based foldamers†
Abstract
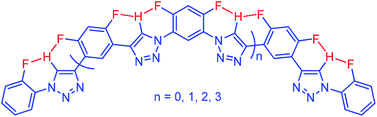
This paper reports the first series of artificial secondary structures that are induced by intermolecular C–H⋯F hydrogen bonding. Oligomers that contain two, four, six, and eight 1,2,3-triazole units have been designed and prepared by connecting the neighbouring 1,2,3-triazole units with 4,6-difluoro-m-phenylene linker(s). Two triphenylmethyl groups are appended at the ends of the backbones to suppress the stacking of the backbones and provide solubility. X-Ray analysis and 1H NMR and two-dimensional 1H–19F heteronuclear Overhauser enhancement spectroscopic (HOESY) experiments support that the 1,2,3-triazole backbones adopt folded or helical conformations due to the formation of continuous three-centred C–H⋯F hydrogen bonding. Quantum chemical calculations reveal that the longest 8-mer foldamer can form a one-turn helical cavity with a diameter of ca. 1.7 nm. Halide anion competition experiments show that the intramolecular C–H⋯F hydrogen bonding is more stable than the well-established intermolecular C–H⋯X− (X = Cl and I) hydrogen bonding.

 Please wait while we load your content...
Please wait while we load your content...