Cp2TiCl-catalyzed highly stereoselective intramolecular epoxide allylation using allyl carbonates†
Abstract
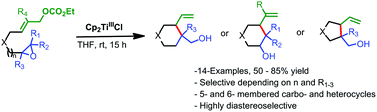
A useful method for the diastereoselective synthesis of vinyl substituted carbo- and heterocycles is described. Highly functionalized structures difficult to achieve by other methodologies are obtained in a single step by this procedure.

 Please wait while we load your content...
Please wait while we load your content...