A non-diazo strategy to cyclopropanation via oxidatively generated gold carbene: The benefit of a conformationally rigid P,N-bidentate ligand†
Abstract
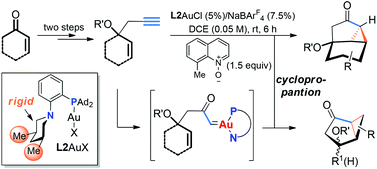
What a trade: diazo ketones for alkynes? By utilizing a conformationally rigid P,N-bidentate ligand, highly efficient intramolecular cyclopropanation reactions using flexible and electronically neutral terminal alkyne substrates are realized via a sterically shielded tri-coordinated gold carbene intermediate. Bicyclic/tricyclic functionalized cyclopropyl ketones are formed in mostly good yields in three steps from readily available enones/enals, which compares favorably with related strategies based on the diazo approach in terms of step economy and operational safety. With this chemistry, we have expanded alkynes as surrogates of α-diazo ketones to cyclopropanation reactions.

 Please wait while we load your content...
Please wait while we load your content...