Synthesis of a naphthalenediimide-based cyclophane for controlling anion–arene interactions†
Abstract
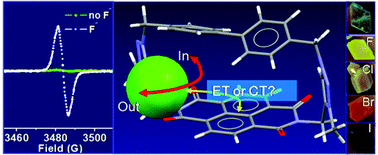
A cationic cyclophane based on a photoactive naphthalenediimide (NDI) moiety and cationic triazolium units has been prepared. This system was employed to control the interactions between anions and the NDI motif and tune the charge transfer properties of the NDI with different anions. High selectivity was observed for fluoride anions, which preferred to interact with the NDI through a SOMO–LUMO-based electronic transition to form NDI radical anions in solution. This cyclophane can crystallize to form a porous lattice which allowed for a gradual leaking of PF6− anions, with replacement by smaller-sized halides. Charge transfer complexes were formed upon replacing the non-nucleophilic hexafluorophosphate anions with halides in the molecular crystals.

 Please wait while we load your content...
Please wait while we load your content...