ε-Methacryloyl-l-lysine based polypeptides and their thiol–ene click functionalization†
Abstract
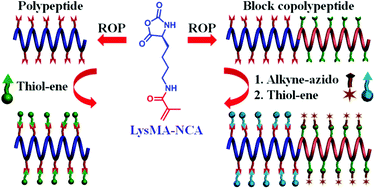
Facile synthesis of biopolymers that facilitate versatile post-polymerization modification is of great interest for biotechnological and biomedical applications. In this study, a methacryloyl-substituted L-lysine N-carboxyanhydride (LysMA-NCA) monomer was designed and synthesized, and methacryloyl-functionalized polypeptides were prepared through the ring opening polymerization (ROP) of the L-lysine-based monomer. The post-polymerization functionalization of the methacryloyl-containing polypeptides with various thiol-containing molecules was achieved with high efficiency through facile radical-mediated thiol–ene chemistry. Moreover, a block copolypeptide bearing both methacryloyl and alkynyl pendants was developed through successive ROP of LysMA-NCA and γ-propargyl-L-glutamate (PPLG-NCA). The sequential modification of the block copolypeptide with hydrophilic and hydrophobic molecules, respectively, was achieved by the successive alkyne–azido and thiol–ene “click” reactions. Overall, the facile synthesis of polypeptides bearing functional substituents and their versatile post-polymerization modification may serve as a useful platform for the development of various functional polymers.


 Please wait while we load your content...
Please wait while we load your content...