Multicomponent isocyanide-based synthesis of reactive styrenic and (meth)acrylic monomers and their RAFT (co)polymerization†
Abstract
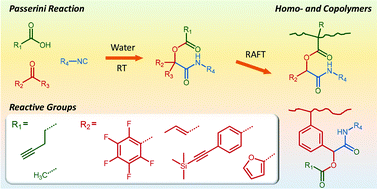
The multicomponent Passerini reaction of aldehydes, carboxylic acids, and isocyanides is used to produce a series of novel reactive (meth)acrylic and styrenic monomers carrying pendant double bond, (trimethylsilyl protected) triple bond, diene, acetate, or pentafluorophenyl functionality. Dichloromethane and water were compared as solvents in the synthesis of 15 different monomers, with water resulting in significantly higher, up to quantitative, isolated yields with minimal purification. Characterization by 1H, 13C, and 19F NMR spectroscopy, FT-IR spectroscopy and mass spectrometry confirmed the synthesis and high purity of the functional α-acyloxycarboxamide products. The monomers are shown to be well suited for the RAFT-synthesis of well-defined homopolymers, statistical copolymers with methyl methacrylate, poly(ethylene glycol) methyl ether methacrylate, and styrene, statistical copolymers produced from two different Passerini-derived monomers, and AB diblock copolymers. SEC-measured polydispersities were generally low, ĐM ≤ 1.29, and 1H NMR spectroscopy confirmed copolymer molar compositions in good agreement with comonomer feed ratios. We expect this synthetic strategy to provide access to a wide range of novel multifunctional materials and demonstrate preliminary postpolymerization modification of a polystyrene derivative by cleavage of its pendent acetate groups and coupling of the dye Methyl Red to the resulting alcohol groups.

 Please wait while we load your content...
Please wait while we load your content...