Copper complexes: the effect of ligands on their photoinitiation efficiencies in radical polymerization reactions under visible light†
Abstract
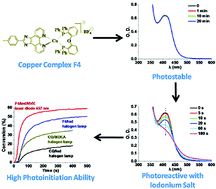
Twenty-three copper complexes (CuC) containing different ligands were synthesized. Most of them exhibited light absorption in the visible range (adapted to LEDs, laser diodes and halogen lamp exposure). The CuC/iodonium salt (Iod), CuC/Iod/N-vinylcarbazole, CuC/alkyl bromide/methyl diethanolamine photoinitiating systems (PISs) allow good monomer conversion profiles for the free radical polymerization of trimethylolpropane triacrylate. Some of them are better than bisacylphosphine oxide or camphorquinone based systems used as references. Real-Time Fourier Transform Infrared spectroscopy and steady state photolysis were used as analytical methods. The results revealed that the ligands of the copper complexes played an important role towards the photoinitiation ability of these PISs. The use of CuC as photocatalysts to initiate thiol–ene reaction is also discussed.

 Please wait while we load your content...
Please wait while we load your content...