Fatty acid-based (bis) 6-membered cyclic carbonates as efficient isocyanate free poly(hydroxyurethane) precursors†
Abstract
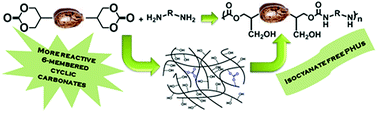
(Bis) 6-membered cyclic carbonates were prepared from methyl 10-undecenoate, which is produced from ricinoleic acid, a main constituent of castor oil. Kinetic studies on these new fatty acid-based 6-membered cyclic carbonates revealed that they are much more reactive than their homologs, 5-membered ones (30 times). Poly(hydroxyurethane)s (PHUs) were then synthesized from these bis 6-membered cyclic carbonates at a temperature as low as room temperature and in the solvent or bulk. Unexpectedly, chemical gels were obtained. The latter were the consequence of side reactions of carbonate ring-opening with the hydroxyl groups of the formed poly(hydroxyurethane)s. Quenching with a large excess of hexylamine enabled the breaking-up of the gel with the formation of urea linkages.

 Please wait while we load your content...
Please wait while we load your content...