Limonene induced chiroptical generation and inversion during aggregation of achiral polyfluorene analogs: structure-dependence and mechanism†
Abstract
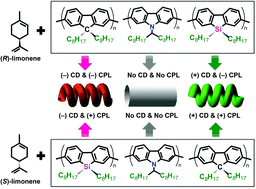
Chirality transfer from biological agents to π-conjugated polymers in the solid state is an attractive method to generate, switch, and amplify chirality, especially when one considers several potent device applications. However, the polymer-structure dependence on the solvent-induced chirality transfer mechanism is not well understood. To elucidate the structural–chiroptical property relationship of the polymer aggregates, poly(9,9-di-n-octylfluorenyl-2,7-diyl) (PF8), poly(9,9-di-n-octylsila-fluorenyl-2,7-diyl) (PSi8), poly(9-(1-octylnonyl)-9H-carbazole-2,7-diyl) (PCz8), P(F8-alt-Si8), P(F8-alt-Cz8), and P(Si8-alt-Cz8) were synthesized. The limonene chirality was efficiently transferred to PF8, PSi8 and P(F8-alt-PSi8) aggregates in CHCl3–limonene–CH3OH tersolvent, but the optical activity was not observable for PCz8, P(F8-alt-PCz8), and P(Si8-alt-PCz8). The reason for the absence of chiroptical activity in Cz8-containing polymers could be that those polymers have stronger polarity compared to limonenes, and thus the interaction between limonene and polymer chains was too weak compared to the interaction among Cz8-containing polymers. Surprisingly, chiroptical inversion in CD spectra between PF8 and PSi8 aggregates was found. More surprisingly, chiroptical inversion between CD and CPL spectra of PSi8 aggregates was observed. The unique chiroptical inversion was attributable to the opposite Mulliken charges between 9-Si in Si8 and 9-C in F8 unit and between Cipso(1) in Si8 and Cipso(1) in F8 unit. Another possible reason for this unexpected chiroptical inversion behaviour is the opposite direction of dipole moments in three stable rotational isomers of the equatorial limonene rotamer.

 Please wait while we load your content...
Please wait while we load your content...