Low bandgap poly(thienylenemethine) derivatives bearing terarylene moieties in the side chains†
Abstract
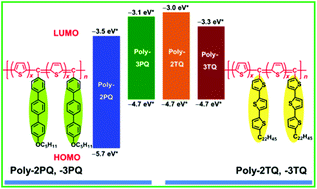
We designed and synthesised low bandgap poly(bithienylenemethine) and poly(terthienylenemethine) derivatives bearing arylene moieties in their side chains. These polymers consisted of alternating benzonoidal and quinoidal structures in the main chains. Arylene moieties were introduced into the side chains to stabilize the quinoidal structure of the polymers because they are anticipated to delocalize π-conjugated electrons of the main chains over the side chains. The polymers were synthesised by polycondensation reactions between arylenes and aryl aldehydes using H2SO4, followed by oxidative dehydrogenation using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). The quinoidal polymers showed absorption bands in the UV-visible and near infrared regions. The optically and electrochemically evaluated bandgaps of quinoidal poly(bithienylenemethine) with the terphenylene moiety in the side chain (Poly-2PQ) were 1.7 and 2.2 eV, respectively, and those of quinoidal poly(terthienylenemethine) with the terphenylene moiety in the side chain (Poly-3PQ) were 0.8 and 1.6 eV, respectively. Similarly, the optical and electrochemical bandgaps of quinoidal poly(bithienylenemethine) with the terthiophene side chain (Poly-2TQ) were 1.5 and 1.7 eV, respectively, and those of quinoidal poly(terthienylenemethine) with the terthiophene side chain (Poly-3TQ) were 0.8 and 1.4 eV, respectively. These low bandgap polymers are regarded as prospective candidate materials for plastic electronics, including organic solar cell devices.

 Please wait while we load your content...
Please wait while we load your content...