Functional α,ω-dienes via thiol-Michael chemistry: synthesis, oxidative protection, acyclic diene metathesis (ADMET) polymerization and radical thiol–ene modification†
Abstract
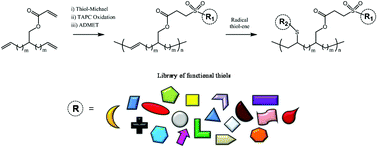
The synthesis of the novel α,ω-diene 2-(undec-10-en-1-yl)tridec-12-en-1-yl acrylate is described. Thiol-Michael coupling of this substrate followed by chemoselective oxidation of the thioether moiety with triazotriphosphorine tetrachloride (TAPC) furnished a suite of functional and symmetrical ADMET-active monomers in a quick and convenient manner. Polymerization of these adducts with Grubbs 1st generation catalyst (RuCl2(PCy3)2CHPh) was demonstrated to high conversion, and quantitative radical initiated thiol–ene modification of the backbone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds was performed to impart additional functionality to each ADMET polymer. These reactions highlight the compatibility of thiol-based click chemistries for the preparation and post-modification of functional ADMET materials.
C bonds was performed to impart additional functionality to each ADMET polymer. These reactions highlight the compatibility of thiol-based click chemistries for the preparation and post-modification of functional ADMET materials.

 Please wait while we load your content...
Please wait while we load your content...