Fast conversion of terminal thiocarbonylthio groups of RAFT polymers to “clickable” thiol groups via versatile sodium azide†
Abstract
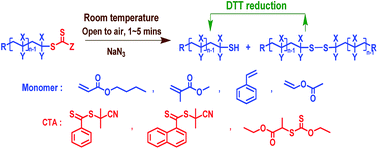
This work reports a facile and fast way of removing thiocarbonylthio end groups of RAFT-made polymers by the utilization of sodium azide (NaN3) without deoxygenation. Within several minutes (1–5 minutes), the terminal thiocarbonylthio group of RAFT-made polybutylacrylate (PBA) was completely removed upon NaN3 treatment as revealed by nuclear magnetic resonance. Careful identification of the chain end structure of the resultant polymer was implemented by matrix-assisted laser desorption ionization time-of-flight mass spectrometry, and the results unambiguously proved that the terminal thiocarbonylthio group was converted to the “clickable” thiol group by NaN3via a nucleophilic process. Polystyrene, poly(methyl methacrylate) and poly(vinyl acetate) prepared by the RAFT technique with different kinds of RAFT agents have also been examined under identical conditions. Similar results were obtained, demonstrating a good universality of this approach. This work provides an alternative and effective approach for removing/modifying thiocarbonylthio end groups of RAFT-made polymers, while producing “clickable” thiol-terminated polymers for many post-modification possibilities.

 Please wait while we load your content...
Please wait while we load your content...