From glycidyl carbonate to hydroxyurethane side-groups in alternating fluorinated copolymers†
Abstract
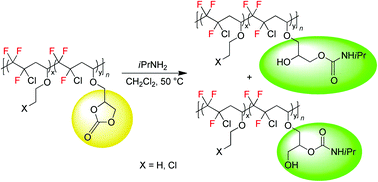
Fluoropolymers bearing five-membered cyclocarbonate pendant groups were synthesized by radical co- or ter-polymerization of chlorotrifluoroethylene (CTFE) with various vinyl ethers [glycerol carbonate vinyl ether (GCVE), ethyl vinyl ether (EVE) and 2-chloroethyl vinyl ether (CEVE)] in good yields. Poly[(CTFE-alt-EVE)-co-(CTFE-alt-GCVE)] and poly[(CTFE-alt-CEVE)-co-(CTFE-alt-GCVE)] terpolymers feature higher molecular weights than poly(CTFE-alt-GCVE) copolymers. Then, the first examples of fluorinated polymers bearing hydroxyurethane moieties (PFHUs) were prepared by the chemoselective reaction of the pendant cyclocarbonates with isopropylamine, leading to primary and secondary hydroxyl dangling groups. The chemical structure of these PFHUs was confirmed by FTIR analysis and 1D (1H, 13C, 135DEPT/APT) and 2D (COSY, HMQC) NMR spectroscopies. Size exclusion chromatography (SEC), differential scanning calorimetry (DSC) and thermogravimetric (TGA) analyses of the PFHUs revealed a higher molecular weight and a decrease in the glass transition and decomposition temperatures, as compared to the parent cyclocarbonate polymers.

 Please wait while we load your content...
Please wait while we load your content...