Highly pH-sensitive polyurethane exhibiting shape memory and drug release†
Abstract
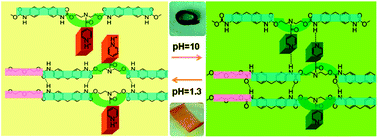
In this study, a highly pH-sensitive polymer is synthesised by introducing pyridine rings into the backbone of polyurethane. The chemical structures of the resulting materials are confirmed by FT-IR and 1H-NMR spectroscopy. To analyse the mechanism of the pH sensitivity of this polymer, its structural transformations under acidic and basic conditions are studied by FT-IR spectroscopy, theoretical calculations and 1H-NMR spectroscopy. We observe that the mechanism of pH responsiveness is the formation of a hydrogen bond interaction between the N atom of the pyridine ring and H–N of urethane in neutral or alkaline environments which is disrupted under acidic conditions due to the protonation of the pyridine ring. The pH-sensitivity is demonstrated by simply adjusting the pH value of the environment, which can act as a switch to control shape memory and drug release. Unlike other systems with thermally sensitive behaviour, the shape memory functionality of this material is independent of temperature, which is dependent only on the variation in the pH of the environment. This strategy provides a potent tool for the design of multifunctional materials based on the physiological environment to fulfil the complex requirements of drug delivery and tissue engineering systems.

 Please wait while we load your content...
Please wait while we load your content...