New dialkoxyamine-trithiocarbonate for the synthesis of multiblock copolymers through in tandem RAFT/NMP†
Abstract
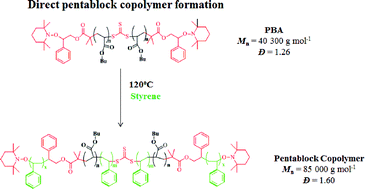
A new strategy for synthesizing multiblock copolymers comprising polystyrene, polyacrylates, poly(N-isopropylacrylamide), etc. is described. The methodology involves the use of a dialkoxyamine-trithiocarbonate (I) to prepare highly end-functionalized polymers through reversible addition–fragmentation chain transfer (RAFT) polymerization at T = 60–70 °C. Under RAFT conditions, the integrity of the alkoxyamine end-group is preserved and the central trithiocarbonate group remains active for the preparation of triblock copolymers. Either the α,ω-dialkoxyamine homopolymer or triblock copolymers were subsequently chain-extended with styrene using nitroxide-mediated radical polymerization (NMP) at T = 120 °C. At this temperature, a tandem polymerization reaction occurs since the trithiocarbonate unit is activated simultaneously. This approach afforded highly pure multiblock copolymers with a narrow molar mass distribution (Đ = 1.3–1.7), which was assessed by size-exclusion chromatography. The diffusion ordered spectroscopy (DOSY) analysis of multiblock copolymers suggests that samples fit well to a single-population model with no evidence of uncoupled blocks detectable. The effectiveness of I for the preparation of a number of multiblock copolymers was demonstrated.

 Please wait while we load your content...
Please wait while we load your content...