Photophysical properties and photochemistry of substituted cinnamates and cinnamic acids for UVB blocking: effect of hydroxy, nitro, and fluoro substitutions at ortho, meta, and para positions†
Abstract
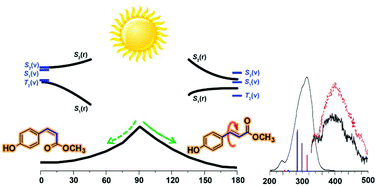
Photophysical properties and photochemistry of various substituted cinnamates and cinnamic acids for ultraviolet B blocking were investigated experimentally and theoretically. This series includes monohydroxy, -nitro, and -fluoro derivatives. The absorption spectra were satisfactorily reproduced by the direct SAC-CI method with respect to the peak position and intensity. The transition character of the low-lying two ππ* and σπ* states for these 18 derivatives was analyzed. The para derivatives have a different transition character of the ππ* transitions compared with those of the ortho and meta derivatives. To elucidate the relaxation mechanism, the emission spectra were observed with oxygen quenching and the photostability was examined experimentally. The calculated radiative lifetimes indicate that the ortho- and meta-substituted derivatives have longer lifetimes for emission than the para derivatives. The potential energy curves of the first and second singlet excited states of the hydroxy derivatives as well as the vertical singlet and triplet transitions were examined to investigate the relaxation qualitatively. The ortho and meta derivatives have an energy barrier or flat surface in S1 resulting in fluorescence, whereas the para derivatives show nonradiative decay without an energy barrier. The para-hydroxy derivative was found to be an excellent UV absorber based on its broad absorption in the UVB/UVA regions, less emission, and higher photostability.

 Please wait while we load your content...
Please wait while we load your content...