A mild preparation of alkynes from alkenyl triflates†
Abstract
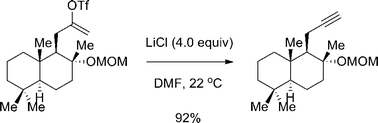
We report herein a protocol for preparing alkynes from alkenyl triflates. Stoichiometric LiCl promotes this transformation in DMF at ambient temperature. A range of terminal and internal alkynes were obtained smoothly. A one-pot procedure of alkyne formation/Cu-mediated Huisgen cycloaddition was developed, which may find use in synthesizing natural product-based probes.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...