1,1-Alkenylboration of diarylphosphino-enynes: convenient synthetic entry to vicinal P/B Lewis pairs at extended conjugated π-frameworks†‡
Abstract
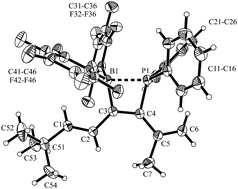
Alkenylboranes R-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-B(C6F5)2 undergo carbon–carbon coupling by means of 1,1-alkenylboration with diarylphosphino-enynes to give substituted conjugated hexatriene derivatives that bear a vicinal pair of B(C6F5)2 Lewis acid and PAr2 Lewis base functionalities at their central carbon portions. A series of six examples was prepared and all compounds were characterized by X-ray diffraction. Consecutive reactions of two selected examples were carried out.
CH-B(C6F5)2 undergo carbon–carbon coupling by means of 1,1-alkenylboration with diarylphosphino-enynes to give substituted conjugated hexatriene derivatives that bear a vicinal pair of B(C6F5)2 Lewis acid and PAr2 Lewis base functionalities at their central carbon portions. A series of six examples was prepared and all compounds were characterized by X-ray diffraction. Consecutive reactions of two selected examples were carried out.
 Please wait while we load your content...
Please wait while we load your content...