A thermodynamic insight into the recognition of hydrophilic and hydrophobic amino acids in pure water by aza-scorpiand type receptors†
Abstract
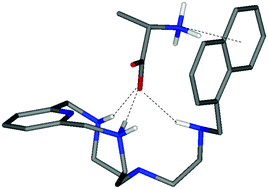
Interactions of different hydrophilic (His, Asp, Glu,) and hydrophobic (Ala, Phe, Tyr, Trp) amino acids in water with a scorpiand aza-macrocycle (L1) containing a pyridine group in the ring and its derivative (L2) bearing a naphthalene group in the tail have been analysed by potentiometric and calorimetric measurements. Theoretical calculations corroborate that major attractive forces that hold the adduct together are hydrogen bonds and salt-bridges, even though other interactions such as π-stacking or NH+⋯π may contribute in the case of hydrophobic amino acids and L2. Calorimetric measurements indicate that the interactions between L1 and the different amino acids are principally driven by entropy, often associated with solvation/desolvation processes.
- This article is part of the themed collection: Supramolecular Chemistry in Water
 Please wait while we load your content...
Please wait while we load your content...