Late-stage diversification of biologically active pyridazinones via a direct C–H functionalization strategy†
Abstract
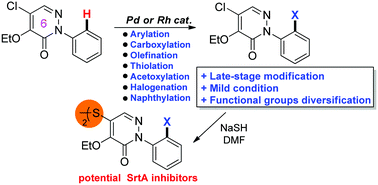
Divergent C–H functionalization reactions (arylation, carboxylation, olefination, thiolation, acetoxylation, halogenation, naphthylation) using a pyridazinone moiety as an internal directing group were successfully established. This approach offers a late-stage, ortho-selective diversification of a biologically active pyridazinone scaffold. Seven series of novel pyridazinone analogues were synthesized conveniently as the synthetic precursors of potential sortase A (SrtA) inhibitors.

 Please wait while we load your content...
Please wait while we load your content...