Pd(ii)-catalyzed C–H arylation of aryl and benzyl Weinreb amides†
Abstract
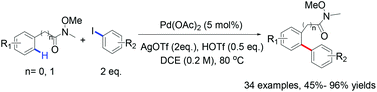
The first example of palladium-catalyzed ortho-C–H arylation of aryl and benzyl Weinreb amides was developed, in which HOTf was used as a key promoter. This method exhibits good functional group tolerance, a broad substrate scope of both Weinreb amides and aryl iodides, high mono-selectivity and mild reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...