Highly enantioselective reaction of 2-oxindoles with (3-indolyl)methanols by cooperative Catalysis of a Lewis acid and organocatalyst†
Abstract
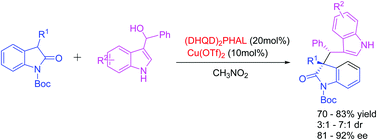
An efficient cooperative biscinchona alkaloid and Lewis acid catalytic system was developed in the enantioselective α-alkylation of 2-oxindoles with (3-indolyl)(phenyl)methanols to provide (2-oxindole)-linker-indole derivatives in good yields (70–83%) with high enantioselectivities (81%–92%).


 Please wait while we load your content...
Please wait while we load your content...