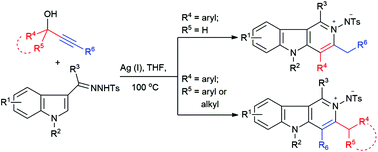
Silver(i)-catalyzed annulation for the regioselective synthesis of N-imino-γ-carbolinium ylides from hydrazones of indole-3-carbonyl derivatives and propargylic alcohols†
Abstract
A regioselective efficient synthetic approach to N-imino-γ-carbolinium ylides via AgOTf-catalyzed iminoannulation has been developed. This transformation proceeds via a silver(I) triflate-catalyzed consecutive Friedel–Crafts reaction/N–C bond formation sequence between the readily available indole derivatives and propargylic alcohols.

 Please wait while we load your content...
Please wait while we load your content...