An efficient synthetic route to 1,3-bis(arylethynyl)isobenzofuran using alkoxybenzocyclobutenone as a reactive platform†
Abstract
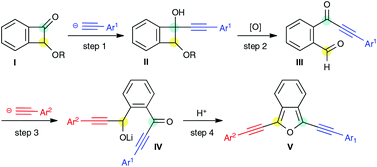
An efficient synthetic method of 1,3-bis(arylethynyl)isobenzofurans is developed. Nucleophilic addition of alkynyllithium to benzocyclobutenone and subsequent oxidative ring cleavage of the four-membered ring gave a keto-aldehyde, which, in turn, accepted the second nucleophile to produce isobenzofurans after acid treatment.

 Please wait while we load your content...
Please wait while we load your content...