Structural insight into the aggregation of l-prolyl dipeptides and its effect on organocatalytic performance†
Abstract
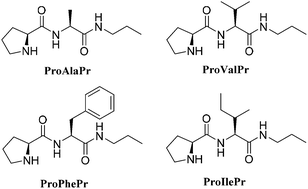
NMR and organocatalytic studies of four dipeptides derived from L-proline are described. Results indicate that important conformational changes around the catalytic L-proline moiety are observed for free dipeptides upon changing the adjacent amino acid. Also, an aggregation process is detected as the concentration increases. The self-association of the dipeptides has been fitted to a cooperative binding model. All the compounds have been assayed as catalysts for the conjugated addition of cyclohexanone to trans-β-nitrostyrene in toluene. In agreement with the structural studies, noticeable changes in the catalytic performance are detected upon changing the catalyst concentration, as the catalyst is activated by self-aggregation.

 Please wait while we load your content...
Please wait while we load your content...