Copper-catalysed α-selective allylic alkylation of heteroaryllithium reagents†
Abstract
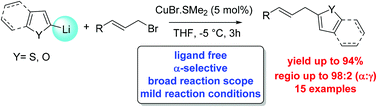
2-Allyl-substituted thiophenes and furans are synthesised efficiently in a direct procedure using 2-heteroaryllithium reagents and allyl bromides and chlorides catalysed by ligand-free copper(I). The reactions take place under mild conditions, with excellent α-selectivity, high functional group tolerance and good yields for the SN2 products.

 Please wait while we load your content...
Please wait while we load your content...