Solid phase synthesis of 1,3,4-oxadiazin-5 (6R)-one and 1,3,4-oxadiazol-2-one scaffolds from acyl hydrazides†
Abstract
Solid phase synthesis of 1,3,4-oxadiazin-5(6R)-one and 1,3,4-oxadiazol-2-one scaffolds from resin-bound acyl hydrazides is described. We demonstrate here that the reactions of resin-bound aryl or hetero-aromatic acyl hydrazides with 2-substituted-2-bromoacetic acids and 4-nitrophenyl chloroformate and subsequent treatment with DIEA lead to intramolecular cyclization reactions to produce six-membered 1,3,4-oxadiazin-5(6R)-ones and five-membered 1,3,4-oxadiazol-2-ones, respectively. We also show that acyl hydrazide-derived 1,3,4-oxadiazol-2-ones may be useful serine hydrolase inhibitors.
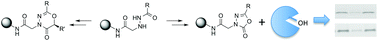

 Please wait while we load your content...
Please wait while we load your content...