Aryl-palladium-NHC complex: efficient phosphine-free catalyst precursors for the carbonylation of aryl iodides with amines or alkynes†
Abstract
A series of aryl-palladium-NHC compounds was prepared according to the reported methods and their catalytic activity in the carbonylation of aryl iodides to synthesize α-keto amides and alkynones was examined. These practical aryl-palladium-NHC complexes have shown highly efficient catalyzed carbonylation and Sonogashira carbonylation reactions, with high turnover number in synthesis of α-keto amides (TON = 4300) and in synthesis of alkynones (TON = 980).
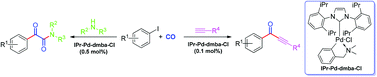

 Please wait while we load your content...
Please wait while we load your content...