A metal-free one-pot cascade synthesis of highly functionalized biaryl-2-carbaldehydes†
Abstract
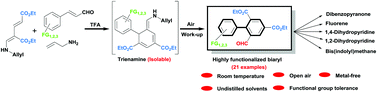
A metal-free one-pot cascade annulation of acyclic substrates dienaminodioate, cinnamaldehydes and allyl amine was achieved for the synthesis of polyfunctional biaryl-2-carbaldehydes. The reaction proceeds at room temperature by a trifluoroacetic acid mediated Diels–Alder pathway. Synthetic applications of the resulting biaryl-2-carbaldehyde have been demonstrated by conversion into an array of diverse molecules with biological and materials chemistry relevance. The present work offers a complementary route to the existing metal mediated cross-coupling methods for the preparation of biaryls.
 Please wait while we load your content...
Please wait while we load your content...