Strained olefin enables triflic anhydride mediated direct dehydrative glycosylation†
Abstract
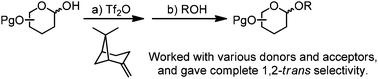
For the first time, we demonstrated that Tf2O mediated direct dehydrative glycosylation was possible simply with strained olefins, and other typical bases were inhibitors of this reaction. We optimized the glycosylation conditions and found that typical benzyl protected 1-OH pyranosyl donors and certain alcohol acceptors were suitable for our glycosylation system. Furthermore, we found that complete 1,2-trans selectivity and a wider acceptor scope could be achieved with 2-O-Bz 3,4,6-tri-O-Bn pyranosyl donors.

 Please wait while we load your content...
Please wait while we load your content...