Gold-catalyzed carboalkoxylations of 2-ethynylbenzyl ethers to form 1- and 3-substituted 2-methoxy-1-H-indenes: Brønsted acids versus gold catalysis†
Abstract
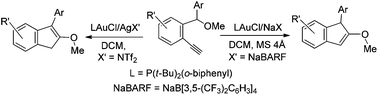
Selective synthesis of 1- and 3-substituted 2-methoxyindenes from the carboalkoxylations of 2-ethynylbenzyl ethers is described; the former is obtained efficiently with P(t-Bu)2(o-biphenyl)AuCl/NaBARF in DCM/MS 4 Å whereas the latter is produced preferably with P(t-Bu)2(o-biphenyl)AuCl/AgNTf2 in pre-dried DCM. Both 1- and 3-substituted 2-indenyl ethers are subjected to ozone oxidations to afford two distinct carbonyl products. Our new data indicate that 1-substituted 2-indenyl ethers are generated from gold catalysts whereas their 3-substituted analogues arise from Brønsted acids.

 Please wait while we load your content...
Please wait while we load your content...