Cross-strand histidine–aromatic interactions enhance acyl-transfer rates in beta-hairpin peptide catalysts†
Abstract
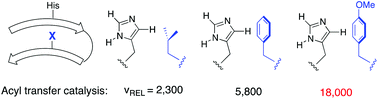
A reactive tagging methodology was used to select the species most reactive to an acylation reagent from a solid phase library of beta hairpin peptides. Hits bearing an electron-rich aromatic residue across strand from a reactive histidine were found to competitively become N-acylated. In addition to displaying rapid N-acylation rates the hit peptide was additionally deacylated in the presence of a nucleophile, thus closing a putative catalytic cycle. Variants of the hit peptide were studied to elucidate both the magnitude (up to 18 000-fold over background, kcat/kuncat = 94 000 000, or 45-fold over Boc-histidine methyl ester) and mechanism of acyl transfer catalysis. A combination of CH–π, cation–π and HisH+–O interactions in the cationic imidazole transition state is implicated in the rate acceleration, in addition to the fidelity of the beta hairpin fold. Moreover, NMR structural data on key intermediates or models thereof suggest that a key feature of this catalyst is the ability to access several different stabilizing conformations along the catalysis reaction coordinate.
- This article is part of the themed collection: Celebrating the 2016 RSC Prize and Award Winners
 Please wait while we load your content...
Please wait while we load your content...