Organocatalysts of oxidative protein folding inspired by protein disulfide isomerase†
Abstract
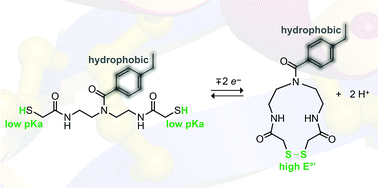
Organocatalysts derived from diethylenetriamine effect the rapid isomerization of non-native protein disulfide bonds to native ones. These catalysts contain a pendant hydrophobic moiety to encourage interaction with the non-native state, and two thiol groups with low pKa values that form a disulfide bond with a high E°′ value.
 Please wait while we load your content...
Please wait while we load your content...