Relative stability of benziporphyrin and naphthiporphyrin tautomers and the emergence of macrocyclic diatropicity†
Abstract
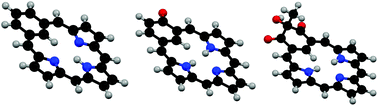
The conformations of a series of benziporphyrins, naphthiporphyrins, oxybenziporphyrins, and related structures were minimized using DFT-B3LYP/6-311++G(d,p). The relative stabilities of the tautomers for each series were calculated using M06-2X and B3LYP-D functionals, and bond lengths were obtained. The diatropic properties of each species were assessed by calculating nucleus independent chemical shifts (NICS) and the related NICSzz values. Although benziporphyrin and naphthiporphyrin tautomers essentially exhibit no aromatic properties, mono- and diprotonated species show weakly diatropic characteristics in agreement with experimental observations. Benziphlorins, intermediates in the MacDonald “3 + 1” synthesis of benziporphyrins, were also examined and tautomers with methylene units adjacent to the benzene ring were shown to be far more stable than tautomers with a CH2 bridge between two pyrrolic units. 2-Hydroxybenziporphyrin was shown to be significantly less stable than the aromatic tautomer oxybenziporphyrin, although diprotonation leads to a species with somewhat reduced diatropicity. Related systems were also analyzed and the results demonstrate that benziporphyrins and naphthiporphyrins range from structures with no measurable macrocyclic aromaticity to strongly aromatic systems that exhibit large diamagnetic ring currents.
 Please wait while we load your content...
Please wait while we load your content...