Anion binding of a neutral bis(cyclopeptide) in water–methanol mixtures containing up to 95% water†
Abstract
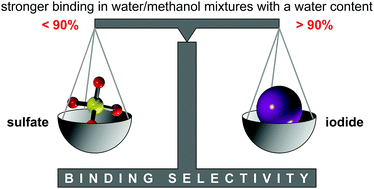
Anion receptor 2b was designed and synthesized, which was structurally derived from a previously described bis(cyclopeptide) 2a comprising two covalently linked cyclic hexapeptide rings with alternating L-proline and 6-aminopicolinic acid subunits. Solubilizing groups attached to the aromatic cyclopeptide subunits of 2b cause a substantial improvement of water solubility with respect to 2a, but have negligible effects on anion binding properties. Thus, anion affinity of 2b could be evaluated in aqueous solvent mixtures in which 2a is not sufficiently soluble, namely in water–methanol with a water content of up to 95 vol%. The solvent-dependent characterization of anion binding showed that the log Ka values of the iodide and sulfate complexes of 2b decrease linearly with increasing water content while the individual contributions of complexation enthalpy and entropy correlate with the solvent composition in a more complex manner. The obtained results provide insight into the factors that control anion affinity and selectivity of these neutral receptors in aqueous media. In addition, they show that substantial anion affinity can be expected even in 100% water.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...