Enantioselective synthesis of α-benzylated lanthionines and related tripeptides for biological incorporation into E. coli peptidoglycan†
Abstract
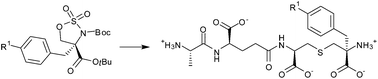
The synthesis of modified tripeptides (S)-Ala-γ-(R)-Glu-X, where X = (R,S) or (R,R) diastereomers of α-benzyl or α-(4-azidobenzyl)lanthionine, was carried out. The chemical strategy involved the enantioselective alkylation of a 4-MeO-phenyloxazoline. The reductive opening of the alkylated oxazolines, followed by cyclization and oxidation, led to four PMB-protected sulfamidates. Subsequent PMB removal, Boc protection and regioselective opening with cysteine methyl ester led to protected lanthionines. These compounds were further converted in a one pot process to the corresponding protected tripeptides. After ester and Boc deprotection, the four tripeptides were evaluated as potential analogues of the natural tripeptide (S)-Ala-γ-(R)-Glu-meso-A2pm. These compounds were evaluated for introduction, by means of the biosynthetic recycling pathway, into the peptidoglycan of Escherichia coli. A successful in vitro biosynthesis of UDP-MurNAc-tripeptides from the tripeptides containing α-benzyl lanthionine was achieved using purified murein peptide ligase (Mpl). Bioincorporation into E. coli W7 did not occur under different tested conditions probably due to the bulky benzyl group at the Cα carbon of the C-terminal amino acid.

 Please wait while we load your content...
Please wait while we load your content...