Asymmetric synthesis of (3S,1′S)-3-(1-amino-2,2,2-trifluoroethyl)-1-(alkyl)-indolin-2-one derivatives by addition of (S)-N-t-butylsulfinyl-3,3,3-trifluoroacetaldimine to 1-(alkyl)-indolin-2-ones†
Abstract
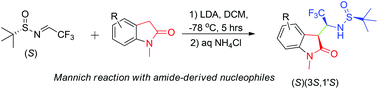
This paper presents the first study of the addition reactions between amide-derived nucleophiles and chiral CF3-containing N-sulfinyl-imines. We demonstrate that enolates of 1-(alkyl)-indolin-2-ones cleanly react with (S)-N-t-butylsulfinyl-3,3,3-trifluoroacetaldimine affording 3-(1-amino-2,2,2-trifluoroethyl)-1-(alkyl)-indolin-2-ones of (3S,1′S) absolute configuration as the major reaction products with synthetically meaningful diastereoselectivity and chemical yields. The reactions were shown to be of general practical application for preparation of various oxindole derivatives in a diastereomerically pure form.

 Please wait while we load your content...
Please wait while we load your content...