A versatile strategy for appending a single functional group to a multifunctional host through host–guest covalent-capture†
Abstract
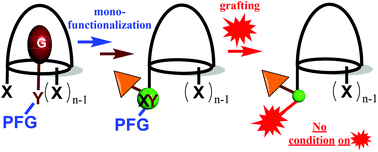
Mono-functionalization of a molecular host is a key step for the development of various efficient systems ranging from supramolecular fluorescent probes to supramolecular catalysts. The presence of several identical reactive groups on the host makes its selective mono-functionalization a challenge. We propose a general two-step strategy to achieve this, based on the receptor properties of the host. A guest bearing two orthogonal functions is first reacted with the host presenting itself a reactive function that is complementary to one of those of the guest. As a result, the host is selectively mono-functionalized by the covalent capture of the guest, which inhibits further reaction of the host. The second function that was present on the guest and which is now covalently linked to the host can be activated in the second step for the grafting of various objects. As a proof of concept, the strategy is described on a calix[6]arene scaffold presenting three identical reactive units. Using Huisgen thermal azide–alkyne cycloaddition for the host–guest covalent-capture step, three examples of post-functionalization are described, allowing cavities bearing a single redox tag, fluorescent probe or polydentate ligand through esterification, Schiff base formation or nucleophilic substitution to be obtained.

 Please wait while we load your content...
Please wait while we load your content...