Synthesis, gelation and topochemical polymerization of meta-linked oligophenylenebutadiynylene derivatives†
Abstract
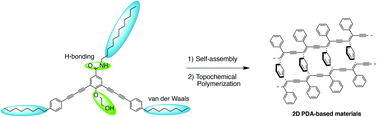
Rational design of meta-linked oligophenylbutadiynylene (OPBD) derivatives was conducted in order to gain insight into their gelation properties and reactivity toward topochemical polymerization to yield polydiacetylenes (PDAs). Different structural parameters influencing the gel formation such as the presence of peripheral 2-hydroxyethoxy side chains and the position of amide functionalities, responsible for intermolecular hydrogen bonds, were studied. Topochemical polymerization of butadiyne units contained within the OPBDs was performed and the resulting PDAs were characterized by electron microscopy and spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...