Anti-hepatitis B virus activities and absolute configurations of sesquiterpenoid glycosides from Phyllanthus emblica†
Abstract
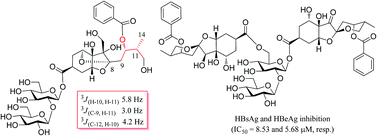
During the process exploring anti-viral compounds from Phyllanthus species, eight new highly oxygenated bisabolane sesquiterpenoid glycoside phyllaemblicins G1–G8 (1–8) were isolated from Phyllanthus emblica, along with three known compounds, phyllaemblicin F (9), phyllaemblic acid (10) and glochicoccin D (11). Phyllaemblicin G2 (2), bearing a tricyclo [3.1.1.1] oxygen bridge ring system, is an unusual sesquiterpenoid glycoside, while phyllaemblicins G6–G8 (6–8) are dimeric sesquiterpenoid glycosides with two norbisabolane units connecting through a disaccharide. All the structures were elucidated by the extensive analysis of HRMS and NMR data. The relative configuration of phyllaemblicin G2 was constructed based on heteronuclear coupling constants measurement, and the absolute configurations for all new compounds were established by calculated electronic circular dichroism (ECD) using time dependent density functional theory. The sesquiterpenoid glycoside dimers 6–9 displayed potential anti-hepatitis B virus (HBV) activities, especially for the new compound 6 with IC50 of 8.53 ± 0.97 and 5.68 ± 1.75 μM towards the HBV surface antigen (HBsAg) and HBV excreted antigen (HBeAg) secretion, respectively.
 Please wait while we load your content...
Please wait while we load your content...