Asymmetric synthesis of axially chiral anilides by Pd-catalyzed allylic substitutions with P/olefin ligands†
Abstract
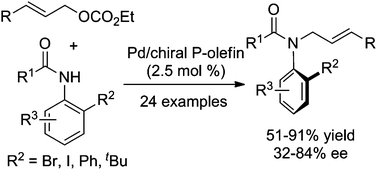
As an attractive class of non-biaryl atropisomeric compounds, C–N axially chiral anilides have received considerable attention, and several methods have been successfully developed for their synthesis. Pd-catalyzed asymmetric allylic amination was proved to be an effective approach for the chiral anilide synthesis, although only moderate enantioselectivity and relatively narrow substrate scope have been achieved in the previous work. Searching for highly efficient methods for the synthesis of axially chiral anilides is therefore of great interest in synthetic and pharmaceutical chemistry. In this paper, a palladium-catalyzed asymmetric allylic substitution of ortho-substituted anilides using phosphorus amidite–olefin ligands was successfully achieved to afford a variety of axially chiral anilides in high yields with up to 84% ee. The absolute configurations of chiral anilides were also determined from X-ray and CD spectra.

 Please wait while we load your content...
Please wait while we load your content...