Formal synthesis of (+)-3-epi-eupomatilone-6 and the 3,5-bis-epimer†
Abstract
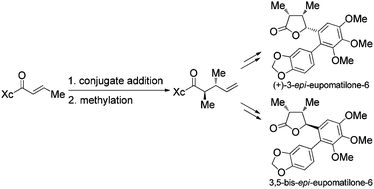
The formal synthesis of (+)-3-epi-eupomatilone-6 (1) and the 3,5-bis-epimer (2) has been accomplished. The key synthetic strategy involved the stereoselective construction of (3R,4S,5R)- and (3R,4S,5S)-trisubstituted γ-butyrolactones 3 and 4 from (2R,3R)-2,3-dimethyl-4-pentenoic acid derivative 7, which was readily obtained via stereoselective conjugate addition of vinylmagnesium chloride to a chiral α,β-unsaturated N-acyl oxazolidinone (Evans’ auxiliary) followed by α-methylation.

 Please wait while we load your content...
Please wait while we load your content...