Studies on copper(i)-catalyzed highly regio- and stereo-selective hydroboration of alkynamides†
Abstract
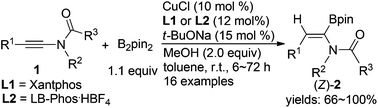
The copper(I)-catalyzed hydroboration of alkynamides with B2pin2 afforded the alkenamide boronates in 66% to nearly quantitative yields with high regio- and stereo-selectivity. It was interesting to note that the regio-selectivity of the reaction is opposite to that observed in the carbometallation reaction of alkynamides, and the resulting alkenyl boronates provided access to α,β-disubstituted (Z)-alkenamides through further elaboration.

 Please wait while we load your content...
Please wait while we load your content...