Palladium-catalyzed direct addition of arylboronic acids to 2-aminobenzonitrile derivatives: synthesis, biological evaluation and in silico analysis of 2-aminobenzophenones, 7-benzoyl-2-oxoindolines, and 7-benzoylindoles†
Abstract
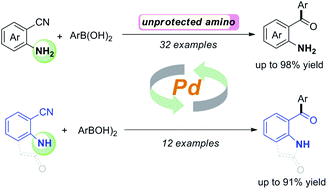
A palladium-catalyzed direct addition of arylboronic acids to unprotected 2-aminobenzonitriles has been developed, leading to a wide range of 2-aminobenzophenones with moderate to excellent yields. The transformation has broad scope and high functional group tolerance. Moreover, 2-oxoindoline-7-carbonitrile and indole-7-carbonitrile were applicable to this process for the construction of 7-benzoyl-2-oxoindolines and 7-benzoylindoles, respectively. Among the compounds examined, compound 4e possessed the most potent anticancer activity against H446 and HGC-27 in vitro, with IC50 values of 0.02 μmol L−1 and 0.09 μmol L−1, respectively, while compound 4a showed the best potent anticancer activity against SGC-7901 with an IC50 value of 0.01 μmol L−1. Furthermore, we also performed in silico molecular docking calculations to investigate the interaction mode and binding affinity between the examined compounds and their tubulin target.

 Please wait while we load your content...
Please wait while we load your content...