Modular synthesis of cyclic cis- and trans-1,2-diamine derivatives†
Abstract
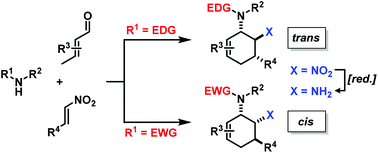
Structurally diverse carbocycles with two vicinal nitrogen-substituents were prepared in expedient three-component reactions from simple amines, aldehydes, and nitroalkenes. trans,trans-6-Nitrocyclohex-2-enyl amines were obtained in a one-pot domino reaction involving condensation, tautomerisation, conjugate addition, and nitro-Mannich cyclisation. Upon employment of less nucleophilic carboxamides, a concerted Diels–Alder cycloaddition mechanism operated to give the corresponding cis,trans-nitrocyclohexenyl amides. Both types of substituted carbocycles offer ample opportunities for chemical manipulations at the core and periphery. Ring oxidation with MnO2 affords substituted nitroarenes. Reduction with Zn/HCl provides access to various trans- and cis-diaminocyclohexenes, respectively, in a straight-forward manner. With enantiopure secondary amines, a two-step synthesis of chiral nitrocyclohexadienes was developed (82–94% ee).

 Please wait while we load your content...
Please wait while we load your content...