The mechanism and regioselectivity of gold(i) or platinum(ii) catalyzed intramolecular hydroarylation to pyrrolopyridinones and pyrroloazepinones†
Abstract
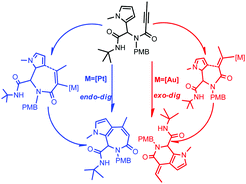
We report here the theoretical analysis of the mechanism and regioselectivity of gold(I) or platinum(II) catalyzed intramolecular hydroarylation to pyrrolopyridinones and pyrroloazepinones. AuPH3+ and PtCl2 have been considered to account for some experimental observations. Our calculation results indicate that in the case of cationic gold the nucleophilic attack of the pyrrole on the activated alkyne occurs in an exo-dig fashion generating a six-membered intermediate, which upon deprotonation and protodeauration forms pyrrolopyridinone. When platinum is used, an endo-dig fashion is observed generating a seven-membered intermediate. After deprotonation and protodeplatination pyrroloazepinone is formed. Whether for exo-dig (gold(I)) or endo-dig (platinum(II)) cyclization, a [1,2]-migration would not be needed.

 Please wait while we load your content...
Please wait while we load your content...