Copper-catalysed direct radical alkenylation of alkyl bromides†
Abstract
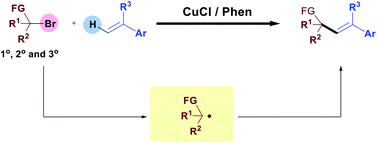
A copper-catalysed direct radical alkenylation of various benzyl bromides and α-carbonyl alkyl bromides has been developed. Compared with the recent radical alkenylations which mostly focused on secondary or tertiary alkyl halides, this transformation shows good reactivity to primary alkyl halides and tertiary, secondary alkyl halides were also tolerated. The key initiation step of this transformation is a copper-induced single-electron reduction of C–Br bonds to generate alkyl radical species.


 Please wait while we load your content...
Please wait while we load your content...