Nickel-catalyzed substitution reactions of propargyl halides with organotitanium reagents†
Abstract
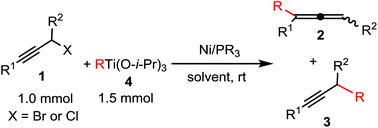
A simple and mild catalytic coupling reaction of propargyl halides with organotitanium reagents is reported. The reaction of propargyl bromide with organo-titanium reagents mediated by NiCl2 (2 mol%) and PCy3 (4 mol%) in CH2Cl2 afforded coupling product allenes in good to excellent yields (up to 95%) at room temperature. However, NiCl2(PPh3)2 was the best catalyst for substituted propargyl halides to yield allenes or alkynes preferentially. On the basis of the experimental results, a possible catalytic cycle has been proposed.

 Please wait while we load your content...
Please wait while we load your content...