First total syntheses of chrestifoline-B and (±)-chrestifoline-C, and improved synthetic routes to bismurrayafoline-A, bismurrayafolinol and chrestifoline-D†‡
Abstract
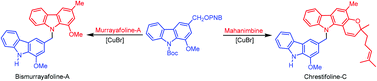
We describe an efficient synthesis of the methylene-bridged biscarbazole alkaloids bismurrayafoline-A, bismurrayafolinol and chrestifoline B–D using an Ullmann-type coupling at the benzylic position.

 Please wait while we load your content...
Please wait while we load your content...