Synthesis, and the antioxidant, neuroprotective and P-glycoprotein induction activity of 4-arylquinoline-2-carboxylates†‡
Abstract
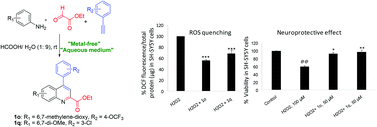
An efficient formic acid catalyzed one-pot synthesis of 4-arylquinoline 2-carboxylates in water via three-component coupling of arylamines, glyoxylates and phenylacetylenes has been described. 4-Arylquinoline 2-carboxylates 1o and 1q displayed significant antioxidant activity as indicated by their Fe-reducing power in the ferric reducing ability of plasma (FRAP) assay. The compounds were found to react directly with hydrogen peroxide, which might be one of the mechanisms of their antioxidant effect. Compounds 1o and 1q effectively quenched H2O2 and amyloid-β-generated reactive oxygen species (ROS) and also displayed significant protection against H2O2-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. Additionally, all compounds exhibited promising P-glycoprotein induction activity in human adenocarcinoma LS-180 cells, indicating their potential to enhance amyloid-β clearance from Alzheimer's brains. Furthermore, all compounds were relatively non-toxic to SH-SY5Y and LS-180 cells (IC50 > 50 μM). The promising antioxidant, ROS quenching, neuroprotective and Pgp-induction activity of these compounds strongly indicate their potential as anti-Alzheimer's agents.

 Please wait while we load your content...
Please wait while we load your content...